HOME > Research Summaries > Development of Clinical Application of Nano Technology for Hearing Impairment to Realize More Precise ABI Positioning and for Delivery of Drugs into the Inner Ear
Research Summaries

Development of Clinical Application of Nano Technology for Hearing Impairment to Realize More Precise ABI Positioning and for Delivery of Drugs into the Inner Ear
Tetsuaki Kawase*, Kiyoshi Oda, Daisuke Yamauchi, Hiroshi Hidaka, Yukio Katori, and Toshimitsu Kobayashi
Professor
Department of Biomedical Engineering, Graduate School of Biomedical Engineering
Email:![]()
1. Introduction
The etiology of sensorineural hearing impairment has not yet been proved, and thus neither its diagnosis nor therapeutic approach in indivisuals so affected has been established. Thus, in the present study, we explore a new diagnostic method and therapy employing nanotechnology in order to 1) enable the clinical use of a new electrostimulation probe for precise intraoperative positioning of an auditory brainstem implant intraoperatively and 2) to establish a system to deliver drugs to the cochlear tissue in patients with sensorineural hearing impairment.
2. Precise Positioning of Auditory Brainstem Implant
An auditory brainstem implant (ABI) is a prosthesis which directly stimulates the cochlear nucleus to restore the “hearing” in patients with bilateral deafness due to cochlear nerve pathology, such as bilateral vestibular schwannomas (neurofibromatosis type II). ABIs have been used in such patients since 1979; however, the auditory performance using an ABI is quite variable among patients [1-3], some patients showing excellent performance, while others do not. One of the important keys to obtain better speech perception performance using an ABI is the position of the electrode. In the present study, we experimentally developed a positioning probe and a way to mark precise position of cochlear nucleus with a navigation system.
2.1. EABR measurement by multi-channel microbipolar electrodes
Guinea pigs were anesthetized with an intramuscular injection of ketamine and xylazine. The multi-channel surface microelectrode (64 channel) was placed on the dorsal cochlear nucleus, which was exposed after the partial removal of the temporal bone. The unequivocal EABR can be elicited by the bipolar stimulation of two of these 64 electrodes (usually the nearest two points, inter-electrode distance = 100μm). The area of cochlear nucleus was estimated by distinguishing the positive electrically evoked auditory brainstem responses (EABRs) from other responses (Figs. 1 and 2).
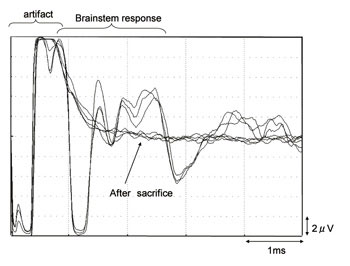
Fig. 1. Typical waves of EABR
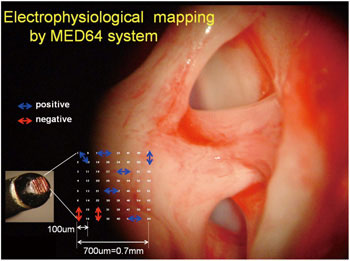
Fig. 2. Multi-channel surface microelectrode. The sixty- four stimulating electrodes are in a 0.7 x 0.7 mm area. Inter-electrode distance is 100μm.
2.2. Precise positioning with navigation guidance based on intraoperative EABR measurement
A single channel contact-type microbipolar stimulation electrode (inter-electrode distance of 200 μm; Eiko company) was used to measure human EABRs during acoustic tumor surgery. The stimulation electrode was connected with SureTrak2 to show the obtained data on the monitor of navigation system (stealthstation; Medtronic) (Fig. 3).
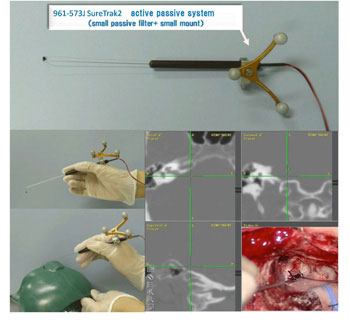
Fig. 3. A single channel contact-type microbipolar stimulation electrode with SureTrak2; EABR measurement points were marked by navigation system.
3. System for Drug Delivery into the Inner Ear
Radical therapy for sensorineural hearing impairment has not yet been established, and only local and systemic administration of steroid administration is effective. Nevertheless neurotrophins (NGF, BDNF, GDNF), Bol-2, NOS inhibiter and glutamate antagonist have recent been experimentally shown to be effective for protecting the inner ear [4,5]. It has also been reported that gene therapy and siRNA may have potential for cureing hereditary hearing loss.
Thus, establishment of a safe and effective drug delivery system (DDS) into the inner ear is needed for clinical application. Because the cochlea is surrounded by temporal bone and isolated from other organs, effective local administration is much safer than systemic administration.
In the present study we research a DDS for the inner ear which has potential for improving the diagnosis and treatment of sensorineural hearing impairment. Research in this area has only recently been undertaken [6,7] and we have also started to explore a DDS and imaging of the inner ear with the use of nano particles.
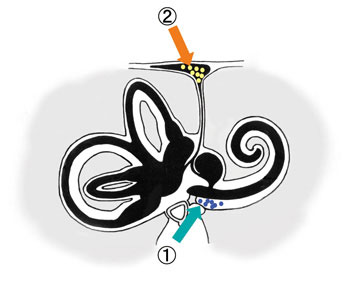
Fig. 4. Round window (1), endolymphatic sac (2). Demonstration of DDS via nano particles.
References
[1] Otto SR, Brackmann DE, Hitselberger WE, Shannon RV and Kuchta J. Multichannel auditory brainstem implant: update on performance in 61 patients. J Neurosurg 96, 1063-1071, 2002.
[2] Nevison B, Laszig R, Sollmann WP, Lenarz T, Sterkers O, Ramsden R, Fraysse B, Manrique M, Rask-Andersen H, Garcia-Ibanez E, Colletti V and von Wallenberg E. Results from a European clinical investigation of the Nucleus multichannel auditory brainstem implant. Ear Hear 23, 170-183, 2002.
[3] Behr R, Muller J, Shehata-Dieler W, Schlake H-P, Helms J, Roosen K, Klug N, Holper BM and Lorens R. The high rate CIS auditory brainstem implant for restoration of hearing in NF-2 patients. Skull Base 17, 91-108, 2007.
[4] Duan ML, Ulfendahl M, Laurell G, Counter AS, Pyykkö I, Borg E and Rosenhall U. Protection and treatment of sensorineural hearing disorders caused by exogenous factors: experimental findings and potential clinical application. Hear Res 169, 169-178, 2002.
[5] Miller JM, Chi DH, O'Keeffe LJ, Kruszka P, Raphael Y and Altschuler RA. Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Int J Dev Neurosci 15, 631-643, 1997.
[6] Nakagawa T and Ito J. Endoscopic modified Lothrop procedure for postoperative frontal mucocele. Acta Otolaryngol Suppl 557, 51-54, 2007.
[7] Tamura T, Kita T, Nakagawa T, Endo T, Kim TS, Ishihara T, Mizushima Y, Higaki M and Ito J. Drug delivery to the cochlea using PLGA nanoparticles. Laryngoscope 115, 2000-2005, 2005.